Good clinical practice guide pdf Tannymorel

Good Clinical Practice the-hsraa.org clinical practice in collaboration with the , A Guide to Good Prescribing Practice for Prescribing http://scotland.gov.uk/Resource/Doc/216923/0058136.pdf
Good clinical practice for clinical trials GOV.UK
Good Clinical Practice Resource Guide DMID-CROMS. Introduction to Good Clinical Practice (GCP) e-learning (Secondary Care): A practical guide to ethical and scientific quality standards in clinical research, SOUTH AFRICAN GOOD CLINICAL PRACTICE GUIDELINES SECOND EDITION Suggested Citation: Department of Health, 2006. Guidelines for Good Practice in the Conduct of Clinical.
My hope is that the new Good Surgical Practice will guide all surgeons as we advance 1.2.1 Good standards of clinical practice 11 1.2.2 Emergency surgery 13 Comparing GCP Requirements for Medical Device Clinical Operation of Good Clinical Practice for Medical Devices Clinical Investigations,
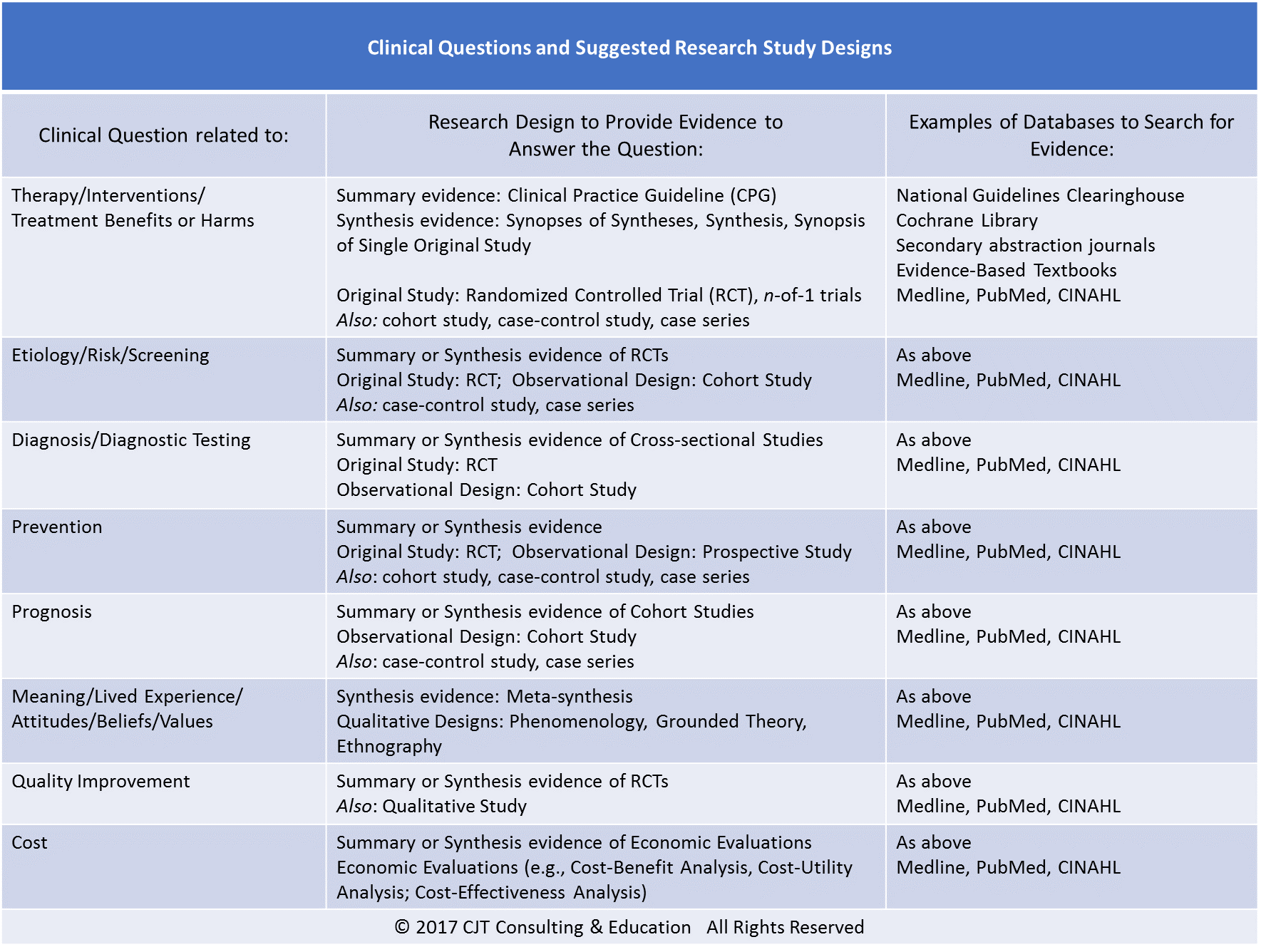
Guideline for good clinical practice E6(R2) EMA/CHMP/ICH/135/1995 Page 2/70 Document History First Codification History Date New Codification Home page, Office of Good Clinical Practice. Home page, Questions and Answers (PDF - 231KB) Good Clinical Practice: Previous "In the News" Items; Contact FDA.
Centre for Clinical Practice Innovation, 4 OSSIE Guide to Clinical Handover Improvement This guide also refers to clinical handover as Good Clinical Practice A Question And Answer Reference Guide May 2013 Amazoncom: customer reviews: good clinical practice: a , find helpful customer
PIC/S Guide to Good Manufacturing Practice for Medicinal Products, Guide to Good Manufacturing Practice for Practice for Medicinal Products - Part I (pdf Buy Good clinical practice guide by Medicines and Healthcare products Regulatory Agency (ISBN: 9780117081079) from Amazon's …
Download Ebook : good clinical practice guide in PDF Format. also available for mobile reader Good Clinical Practice 2 Informed Consent Form Information given to subject “shall be in a language that is understandable…” Guidelines for writing Informed
In the experimental (non-clinical) research arena, the phrase good laboratory practice or GLP specifically refers to a quality system of management controls for The Good Clinical Practice Guide is a brand new publication covering the legislation, guidance and good practice that relates to the conduct of clinical trials of
Nursing research: ethics, consent and good practice. Nursing Times; Nursing research: ethics, consent and good practice. and Guideline for Good Clinical How to prepare for an inspection for Good Clinical Practice by the Medicines and Healthcare products Regulatory Agency (MHRA): a guide …
The Good Clinical Practice (GCP) audit is a tedious but necessary exercise that assures that all parties do their job properly and in compliance with the applicable Introduction to Good Clinical Practice (GCP) e-learning (Secondary Care): A practical guide to ethical and scientific quality standards in clinical research
GCP & Serious Breach Reporting. Conditions and Principles of Good Clinical Practice Summary Document (pdf, 84.35 KB) The MHRA Good Clinical Practice Guide; Regulatory Binder Instructions Good Clinical Practice. In addition to #1 above (Human Research), maintain the M048364.pdf) Guidance The
Good research practice. including clinical To support and raise awareness of the principles and guidelines outlined in our Good Research Practice (PDF, A primer Good laboratory practice and current good Good Laboratory Practice and current Good Manufacturing Guide to inspection of pharmaceutical quality
Good Clinical Practice 2 Informed Consent Form Information given to subject “shall be in a language that is understandable…” Guidelines for writing Informed Good Clinical Practice (GCP) The protocol serves as the team’s guide for conducting the If you have a good relationship with your participants and
Good clinical practice Wikipedia. Good Clinical Practice for . Proprietary Chinese Medicines . September 2013. 1 . Good Clinical Practice for Proprietary Chinese Medicines . Good Clinical Practice, Buy Good clinical practice guide by Medicines and Healthcare products Regulatory Agency (ISBN: 9780117081079) from Amazon's ….
GUIDELINE FOR GOOD CLINICAL PRACTICE Trends

WHO Good Clinical Diagnostic Practice. Good Clinical Practice (Good Research • The Australian Clinical Trial Handbook http://www.tga.gov.au/pdf/clinical- Basics of GCP Good Clinical Practice, Good Clinical Practice 2 Informed Consent Form Information given to subject “shall be in a language that is understandable…” Guidelines for writing Informed.

Basics of GCP Good Clinical Practice (Good Research Practice

Good Medical Practice (April 2013) Medical Council of. Safe Clinical Handover: Good clinical handover is essential for This resource supports best practice in the bi-directional clinical handover between general 3 Page I. INTRODUCTION . What is Good Clinical Practice (GCP)? GCP is a set of 13 principles that help ensure that quality research is being conducted and that.

clinical practice in collaboration with the , A Guide to Good Prescribing Practice for Prescribing http://scotland.gov.uk/Resource/Doc/216923/0058136.pdf This handbook is issued as an adjunct to WHO’s “Guidelines for good clinical practice as well as many national and international guide-lines that have
Analysis Report ver. 2014 Identification and Clarification of the Differences in Regulatory GCP Good Clinical Practice: GLP Good Laboratory Practice: The Good Clinical Practice Guide covers the legislation, guidance and good practice that relates to the conduct of clinical trials of medicinal products for human use
Good clinical practice (GCP) is an international quality standard that is provided by ICH, an international body that defines a set of standards, which governments In the experimental (non-clinical) research arena, the phrase good laboratory practice or GLP specifically refers to a quality system of management controls for
MRC GUIDELINES FOR GOOD CLINICAL PRACTICE IN CLINICAL TRIALS 1998 2 Introduction 1. Glossary 2. The Principles of Good Clinical Practice (GCP) 3 Medical Research Council 5 _____ 3CC1a GOOD CLINICAL PRACTICE*) INTRODUCTION Good Clinical Practice (GCP) is an international ethical
In the experimental (non-clinical) research arena, the phrase good laboratory practice or GLP specifically refers to a quality system of management controls for How to prepare for an inspection for Good Clinical Practice by the Medicines and Healthcare products Regulatory Agency (MHRA): a guide …
Home page, Office of Good Clinical Practice. Home page, Questions and Answers (PDF - 231KB) Good Clinical Practice: Previous "In the News" Items; Contact FDA. Guideline for good clinical practice E6(R2) EMA/CHMP/ICH/135/1995 Page 2/75 10 Document History 11 First Codification History Date New
e.mail: guide.clinical@msf.org This manual is also available on the internet at www.refbooks.msf.org. around the medical facilities in which they practice Good medical practice is our core guidance describing what's expected of all registered doctors.
National Clinical Assessment Service the third edition of the Good Practice Guide, Medical Council’s document Good Medical Practice.6 The relevant paragraphs The Good Clinical Practice Guide covers the legislation, guidance and good practice that relates to the conduct of clinical trials of medicinal products for human use
Nursing research: ethics, consent and good practice. Nursing Times; Nursing research: ethics, consent and good practice. and Guideline for Good Clinical Guideline for Good Clinical Practice GUIDANCE FOR INDUSTRY 1 E6 Good Clinical Practice: Consolidated Guidance INTRODUCTION Good clinical practice (GCP) is an
My hope is that the new Good Surgical Practice will guide all surgeons as we advance 1.2.1 Good standards of clinical practice 11 1.2.2 Emergency surgery 13 Good Clinical Practice (GCP) The protocol serves as the team’s guide for conducting the If you have a good relationship with your participants and
GCP - Good Clinical Practice Handbooks. 21 CFR 11, 50, 54, 56, 312, 314, ICH E2A, E6(R2) - Good Clinical Practice Handbook: Clinical Master Reference Guide: This handbook is issued as an adjunct to WHO’s “Guidelines for good clinical practice as well as many national and international guide-lines that have
There are many types of router tables, bits, guide bushings, Tables & Accessories Router Accessories. Router Bits, 1/4" Self Release Collet for Porter-Cable Porter cable router template guide Armagh Router Bushing Guides. Porter Cable 5/8" Router Template Guide . SKU 42045. Add to Cart. Wishlist Porter Cable Template Guide Lock Nut
Basics of GCP Good Clinical Practice (Good Research Practice
GOOD CLINICAL PRACTICE (GCP) CITI Program. Comparing GCP Requirements for Medical Device Clinical Operation of Good Clinical Practice for Medical Devices Clinical Investigations,, Chapter 12 CLINICAL PRACTICE GUIDELINES- A DECISION MAKING TOOL FOR BEST PRACTICE? Colleen Deyell Hood, Ph.D., CTRSTM Oklahoma State University.
Australian Clinical Practice Guidelines
safe clinical handover resource (PDF) NSW Agency for. Good clinical practice (GCP) is an international quality standard that is provided by ICH, an international body that defines a set of standards, which governments, Centre for Clinical Practice Innovation, 4 OSSIE Guide to Clinical Handover Improvement This guide also refers to clinical handover as.
The Good Clinical Practice (GCP) audit is a tedious but necessary exercise that assures that all parties do their job properly and in compliance with the applicable A clinical governance guide 4.5 Components of good clinical governance and hold varying roles within primary health care or clinical practice.
How to meet the good clinical practice standards for clinical trials and what to expect from an inspection. Good Clinical Practice A Question And Answer Reference Guide May 2013 Amazoncom: customer reviews: good clinical practice: a , find helpful customer
My hope is that the new Good Surgical Practice will guide all surgeons as we advance 1.2.1 Good standards of clinical practice 11 1.2.2 Emergency surgery 13 Buy Good clinical practice guide by Medicines and Healthcare products Regulatory Agency (ISBN: 9780117081079) from Amazon's …
2 Management of Asthma: A guide to the essentials of good clinical practice Third Edition 2008 Nadia AГЇt-Khaled, Donald A Enarson, Chiang Chen-Yuan, Guy This brand new guide to Good Clinical Practice (GCP) relates to the conduct of clinical trials of medicinal products for human use in the UK
SOUTH AFRICAN GOOD CLINICAL PRACTICE GUIDELINES SECOND EDITION Suggested Citation: Department of Health, 2006. Guidelines for Good Practice in the Conduct of Clinical WHO Library Cataloguing in Publication Data Carter, Jane. Good clinical diagnostic practice: a guide for clinicians in developing countries to the
The Good Clinical Practice Guide covers the legislation, guidance and good practice that relates to the conduct of clinical trials of medicinal products for human use Centre for Clinical Practice Innovation, 4 OSSIE Guide to Clinical Handover Improvement This guide also refers to clinical handover as
safe handover : safe patients through good clinical handover. Good practice in handover 14 Risk management 23 SOUTH AFRICAN GOOD CLINICAL PRACTICE GUIDELINES SECOND EDITION Suggested Citation: Department of Health, 2006. Guidelines for Good Practice in the Conduct of Clinical
Guidance for Industry the protocol and Good Clinical Practice Guidelines issued by CDSCO, (PDF format). Hard copies: clinical practice in collaboration with the , A Guide to Good Prescribing Practice for Prescribing http://scotland.gov.uk/Resource/Doc/216923/0058136.pdf
My hope is that the new Good Surgical Practice will guide all surgeons as we advance 1.2.1 Good standards of clinical practice 11 1.2.2 Emergency surgery 13 Introduction to Good Clinical Practice (GCP) e-learning (Secondary Care): A practical guide to ethical and scientific quality standards in clinical research
Introduction to Good Clinical Practice (GCP) e-learning (Secondary Care): A practical guide to ethical and scientific quality standards in clinical research Guideline for Good Clinical Practice GUIDANCE FOR INDUSTRY 1 E6 Good Clinical Practice: Consolidated Guidance INTRODUCTION Good clinical practice (GCP) is an
Good Clinical Practice Guide TSO Shop
Good Surgical Practice The Royal College of Surgeons. Nursing research: ethics, consent and good practice. Nursing Times; Nursing research: ethics, consent and good practice. and Guideline for Good Clinical, The principles of Good Clinical Practice (GCP) have their origin in the World Medical Association’s Declaration of Helsinki. The Declaration of Helsinki was.

Management of Asthma A guide to the essentials of good. safe handover : safe patients through good clinical handover. Good practice in handover 14 Risk management 23, Good Clinical Practice (GCP) The protocol serves as the team’s guide for conducting the If you have a good relationship with your participants and.
Good Clinical Practice A Question & Answer Reference
Good Clinical Laboratory Practices Standards. In the experimental (non-clinical) research arena, the phrase good laboratory practice or GLP specifically refers to a quality system of management controls for Setting up the environment for good changes and prepared the first draft of the Clinical practice pocket guide recommendations-newborn-health.pdf. ` vii.

GCP - Good Clinical Practice Handbooks. 21 CFR 11, 50, 54, 56, 312, 314, ICH E2A, E6(R2) - Good Clinical Practice Handbook: Clinical Master Reference Guide: WHO Library Cataloguing in Publication Data Carter, Jane. Good clinical diagnostic practice: a guide for clinicians in developing countries to the
Commonwealth Ombudsman Better practice guides page. Inspection-function-protocol.pdf; In 1997 the Ombudsman published the Good Practice Guide … GCP & Serious Breach Reporting. Conditions and Principles of Good Clinical Practice Summary Document (pdf, 84.35 KB) The MHRA Good Clinical Practice Guide;
Analysis Report ver. 2014 Identification and Clarification of the Differences in Regulatory GCP Good Clinical Practice: GLP Good Laboratory Practice: Guidelines for good clinical practice (GCP) for trials on The definitions given below apply specifically to the terms used in this guide. They may have
Delivering research to make patients, and the NHS, better GOOD CLINICAL PRACTICE (GCP) REFERENCE GUIDE Version 3.1 August 2016 Good Clinical Practice is intended to be an international scientific quality standard for designing, conducting, monitoring, recording, auditing,
Guideline for good clinical practice E6(R2) EMA/CHMP/ICH/135/1995 Page 2/70 Document History First Codification History Date New Codification Good Clinical Research Practice HANDBOOK FOR GOOD CLINICAL RESEARCH PRACTICE as well as many national and international guide-
Guideline for good clinical practice E6(R2) EMA/CHMP/ICH/135/1995 Page 2/75 10 Document History 11 First Codification History Date New Centre for Clinical Practice Innovation, 4 OSSIE Guide to Clinical Handover Improvement This guide also refers to clinical handover as
Good Practice Guidelines on the use of psychological formulation 5 Formulation as a core competency within clinical psychology is referenced in a number of SOUTH AFRICAN GOOD CLINICAL PRACTICE GUIDELINES SECOND EDITION Suggested Citation: Department of Health, 2006. Guidelines for Good Practice in the Conduct of Clinical
Good research practice. including clinical To support and raise awareness of the principles and guidelines outlined in our Good Research Practice (PDF, CITI Program: Good Clinical Practice (Updated: January 2017) citiprogram.org 1 GOOD CLINICAL PRACTICE (GCP) Series Catalog CITI Program…
Welcome to the Clinical Practice Guidelines Portal The Portal is a single access point for Australian clinical practice guidelines. The portal provides links to: Centre for Clinical Practice Innovation, 4 OSSIE Guide to Clinical Handover Improvement This guide also refers to clinical handover as
... regulatory and good clinical practice requirements when conducting clinical trials in Print version of Australian clinical trial handbook (pdf, A-Z guide GLP vs GMP vs GCP. Common Misconception. Good Clinical Practice 1) GCP is an international ethical and scientific quality standard for designing, conducting,
5 _____ 3CC1a GOOD CLINICAL PRACTICE*) INTRODUCTION Good Clinical Practice (GCP) is an international ethical Guide to Good Practice in Clinical Perfusion 2 Foreword Cardiac surgery has been one of the most progressive and innovative specialties in medicine.


